
1.General
The power fittings are usually made by steel/iron, aluminum or copper. Considering cost and physical properties, steel/iron materials are to be made as pole accessories and connections in insulator strings. In order to prevent corrosion in decades of service life, galvanizing is an effective way which has been widely used globally.
2.Performance of Hot-dip galvanizing
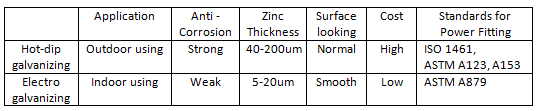
Hot-dip galvanizing is a method in which steel components are dipped in molten zinc to obtain a metal coating. Hot-dip galvanizing has good coverage, dense coating and no organic inclusions. The mechanism of zinc resistance to atmospheric corrosion includes mechanical protection and electrochemical protection. Under atmospheric corrosion conditions, there are protective films of ZnO, Zn(OH)2 and basic zinc carbonate on the surface of the zinc layer, which can slow down the corrosion of zinc to a certain extent. The layer protective film (also called white rust) is damaged and a new layer is formed.
There is another way of galvanizing which called electro galvanizing, it is coated by electrochemical process, its coating thickness is thinner and therefore less protection. The electro galvanizing is not suitable for outdoor fittings.
Characteristic Sheet
3.Principle of Hot-dip galvanizing
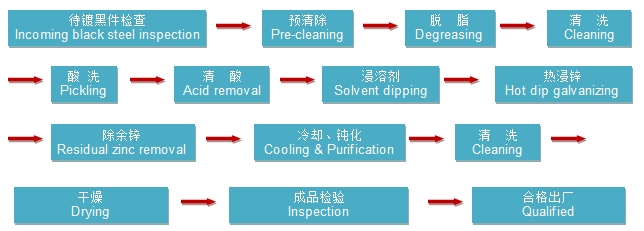
The formation process of the hot-dip galvanizing layer is the process of forming an iron-zinc alloy between the iron matrix and the outermost pure zinc layer. The iron-zinc alloy layer is formed on the surface of the workpiece during hot-dip coating, which makes the iron and pure zinc layer very close. Good combination, the process can be simply described as: when the iron workpiece is immersed in molten zinc, a solid solution of zinc and α iron (body core) is first formed on the interface. This is a crystal formed by dissolving zinc atoms in the base metal iron in a solid state. The two metal atoms are fused, and the attraction force between the atoms is relatively small. Therefore, when zinc reaches saturation in the solid solution, the two element atoms of zinc and iron diffuse each other, and the zinc atoms that have diffused (or infiltrated) into the iron matrix migrate in the matrix lattice, and gradually form alloys with iron, and diffuse The iron and zinc in the molten zinc form an intermetallic compound FeZn13, which sinks into the bottom of the hot-dip galvanizing pot, which is called zinc dross. When the workpiece is removed from the zinc immersion solution, a pure zinc layer is formed on the surface, which is a hexagonal crystal. Its iron content is not more than 0.003%.
4.Step of Hot-dip galvanizing
5. Sample of Hardwares
There are so many fittings which are plated by zinc. Actually all the steel/iron materials are requested to be coated by hot dip galvanizing. Here are some normal fittings with hot dip galvanizing for reference:
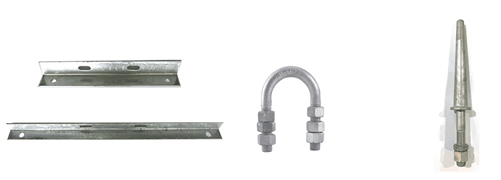
Support brace U Bolt Steel Pin
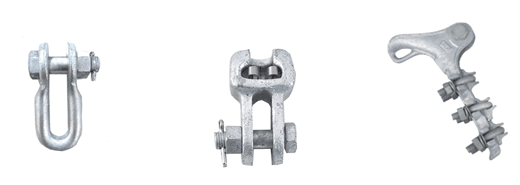
Shackle Socket Steel Clamp